Merilife MeRes100
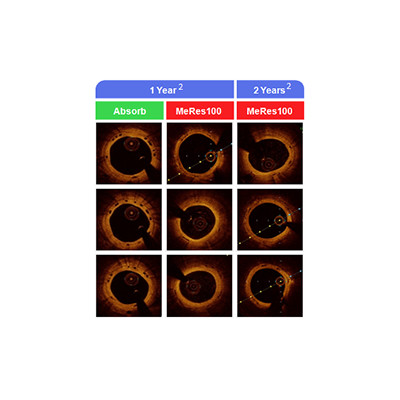
The next generation technology in coronary stenting has arrived in form of BioResorbable Vascular Scaffolds (BRS). The concept of cameo appearance (implantation) of a stent for acute benefits and its eventual disappearance in order to free the vessel of a metal cage is not just enticing; it appears to be the ultimate goal in achieving biomimicry in coronary stenting. However, the research on BioResorbable Scaffolds (BRS) has been long drawn over the past two decades. Technological barriers and limitations into our insight regarding biomaterial science have further slowed down the progress. While several technologies in BRS are under development, the initial results driven by 1st generation technologies are encouraging and path breaking.
Low long term MACE, lumen enlargement, restoration of vasomotion in scaffolded segment are some of the currently understood benefits of BRS technology. Several platforms currently use the primary scaffold backbone as poly-L-lactide (PLLA) which insitu undergoes hydrolysis resulting in a mass loss and further cleavage of the long chains into easily metabolized lactic acid. The resultant conversion to CO2 and H2O via Kreb’s cycle is now well catalogued. The process is completed in a range of 24 – 36 months. An anti-proliferative coating optimally ensures minimization of restenosis.
Amongst several BRS’ under development worldwide, MeRes100 – Sirolimus Eluting BioResorbable Vascular Scaffold System is fully developed by Meril R&D in India and is a next generation BRS. The device is a clever iteration with a low profile delivery system; desirably thinner struts (100µm) and is easier to deploy since it has 3 radiopaque markers at each end, which facilitate ease of procedure.
MeRes100 comprises of the following components:
- A balloon expandable BRS made from polymer – poly-L-lactide (PLLA)
- A top-coat comprising of an anti proliferative agent – Sirolimus (1.25 μg/mm2) eluting from PDLLA, a biodegradable formulation
- A rapid exchange PTCA balloon catheter which acts as the scaffold delivery system
Department:
Brand:
Model Num:


